PANZYGA | FDA - U.S. Food and Drug Administration. PANZYGA. STN: 125587. Proper Name: Immune Globulin Intravenous (Human)-ifas. Tradename: PANZYGA. Manufacturer: Octapharma Pharmazeutika Produktionsges.m.b.HIndication panzyga. Indication: for the . panzyga. PANZYGA® (Immune Globulin Intravenous (Human)-ifas, 10% solution . panzyga
Panzyga
κολπο να κερδιζεις στο κινο
PANZYGA | FDA - U.S. Food and Drug Administration. PANZYGA. STN: 125587. Proper Name: Immune Globulin Intravenous (Human)-ifas. Tradename: PANZYGA. Manufacturer: Octapharma Pharmazeutika Produktionsges.m.b.HIndication panzyga. Indication: for the . panzyga. PANZYGA® (Immune Globulin Intravenous (Human)-ifas, 10% solution . panzyga
πως βαφω τοιχο με υγρασια
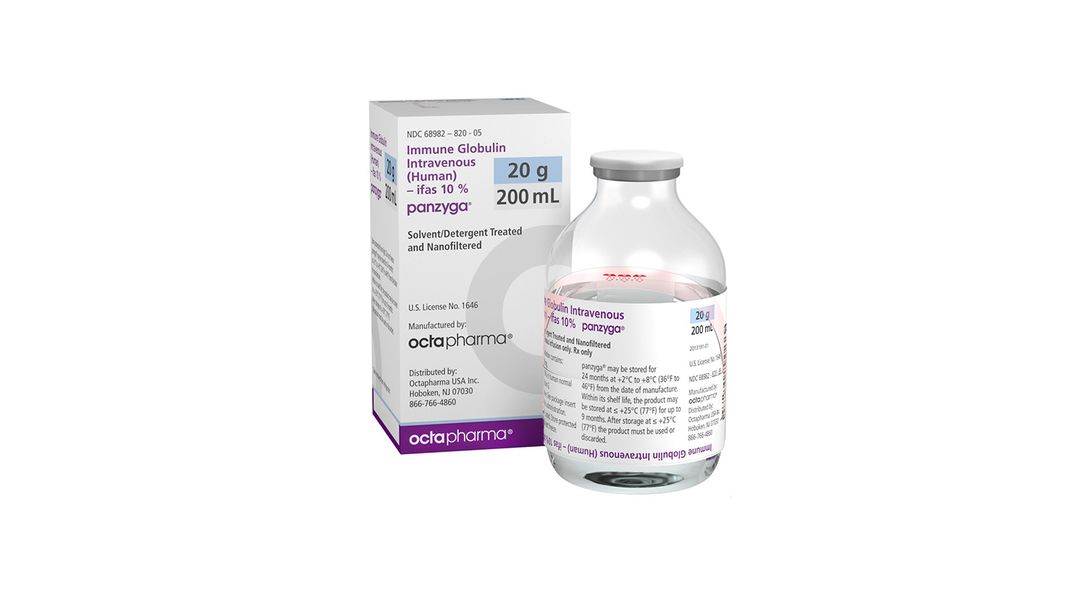
. PANZYGA is made from human plasma and may contain infectious agents, e.g. viruses and theoretically, the Creutzfeldt-Jakob disease agent. Adverse Reactions PI - The most common adverse reactions (>5% study subjects) were headache, nausea, fever, fatigue, and abdominal pain. cITP in adults - The most common adverse reactions (>5% study .. Panzyga: Uses, Side Effects, Dosage & Reviews - GoodRx. Panzyga is a replacement for immunoglobulin G (IgG). IgG is an antibody that your immune system makes to help protect against organisms like viruses, bacteria, and fungi. Certain medical conditions can cause your immune system to be too active or too inactive. The exact way Panzyga works to treat these medical conditions isnt fully known.. Panzyga: Package Insert - Drugs.com. PANZYGA contains mainly immunoglobulin G (IgG) with a broad spectrum of antibodies against various infectious agents reflecting the IgG activity found in the donor population. PANZYGA which is prepared from pooled material from not less than 1000 donors, has an IgG subclass distribution similar to that of native human plasma. .. PANZYGA | Pfizer. PANZYGA (immune globulin intravenous, human - ifas) This product information is intended only for residents of the United States. for Healthcare professionals: panzyga. PANZYGA® - Octapharma. PANZYGA® is a liquid preparation of immune globulin (IVIg) for the treatment of autoimmune diseases and other conditions. It is supplied in different dosages and is only available with a doctors prescription. Learn more about PANZYGA® and its prescribing information. panzyga
jktslot 78
. PANZYGA® (Immune Globulin Intravenous (Human)-ifas, 10% solution .. PANZYGA is a liquid medicine for infusion that contains immunoglobulin G (IgG), which are proteins that help fight infection. It is made from human plasma that is donated by healthy people and contains antibodies. For patients with PI, PANZYGA helps replace the missing antibodies in the body.
本多忠勝 死因
. Panzyga (Immune Globulin Injection (IV)) - Drugs.com. Tell all of your health care providers and lab workers that you take Panzyga (immune globulin injection (IV)). This medicine is made from human plasma (part of the blood) and may have viruses that may cause disease. This medicine is screened, tested, and treated to lower the chance that it carries an infection. panzyga. Panzyga Intravenous: Uses, Side Effects, Interactions, Pictures panzyga. - WebMD. Find patient medical information for Panzyga intravenous on WebMD including its uses, side effects and safety, interactions, pictures, warnings and user ratings.. U.S. FDA Approves PANZYGA - Business Wire. PANZYGA is contraindicated in patients who have a history of severe systemic hypersensitivity reactions, such as anaphylaxis, to human immunoglobulin and in IgA-deficient patients with antibodies .. Panzyga (Intravenous) - Drugs.com panzyga. Uses for Panzyga panzyga. Immune globulin-ifas injection contains antibodies that make your immune system stronger. It is used for patients who have primary humoral immunodeficiency (PI), including congenital agammaglobulinemia, common variable immunodeficiency, X-linked agammaglobulinemia, Wiskott-Aldrich syndrome, and other severe combined immune system problems.. U.S. FDA Approves PANZYGA® for the Treatment of Adults with panzyga

sa kushton te blesh nje yll
. Updated NACI recommendations for measles post-exposure prophylaxis panzyga. For IVIg administration, 400 mg/kg is a standard dosage that is within the indicated range of Ig replacement therapy for patients with primary immunodeficiency according to Canadian product monographs for Gammagard ® (18,19), Gamunex , IGIVnex , Privigen ® and Panzyga ® which are the IVIg products available in Canada through Canadian Blood .. Immune Thrombocytopenia | NEJM panzyga. Immune thrombocytopenia (ITP) is an autoimmune disease characterized by isolated thrombocytopenia. Patients may be asymptomatic at presentation or they may present with mild mucocutaneous to life .. The indications and safety of polyvalent immunoglobulin for post .. Introduction panzyga. Passive immunization is the transfer of antibodies to a recipient. It occurs during pregnancy, with antibodies being actively transported across the placenta and affording the neonate protection against communicable diseases to which the mother is immune for a number of months post-delivery. 1 Injection or infusion of antibodies from donors is also a form of passive immunization .. October 2023 - When will the patents on PANZYGA expire, and when will .. 1) High Certainty: US Patents for PANZYGA Derived from Brand-Side Litigation. No patents found based on brand-side litigation. 2) High Certainty: US Patents for PANZYGA Derived from Company Disclosures. No patents found based on company disclosures. 3) Low Certainty: US Patents for PANZYGA Derived from Patent Text Search. What does Zonyga mean? - Definitions.net. Definition of Zonyga in the Definitions.net dictionary panzyga. Meaning of Zonyga panzyga. What does Zonyga mean? Information and translations of Zonyga in the most comprehensive dictionary definitions resource on the web.. What Does The Name Panzyga Mean? - The Meaning of Names panzyga. For the Name Panzyga; Related Names; Pronunciation; Meanings and Origins; Fun Facts; What Panzygas Have Visited This Page? Name Poster; Keep scrolling for more. Embed panzyga. Panzyga . Meaning of Panzyga; Classmate Finder; Find Family Tree; Free Dating Sites; Gender: Unknown First Name <100 in the U.S. since 1880. Last Name <100. PANZYGA (immune globulin intravenous- human solution - DailyMed. PANZYGA contains mainly immunoglobulin G (IgG) with a broad spectrum of antibodies against various infectious agents reflecting the IgG activity found in the donor population. PANZYGA which is prepared from pooled material from not less than 1000 donors, has an IgG subclass distribution similar to that of native human plasma. . panzyga. PDF Billing and Coding Information - pfizeriguideresources. for PANZYGA. PANZYGA is a solution for infusion to be administered intravenously (IV) in an infusion center, physicians office, or at home by a trained healthcare provider. International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) Diagnosis Codes1. PANZYGA® (Immune Globulin Intravenous (Human)-ifas, 10% solution .. PANZYGA storage and handling 1. PANZYGA can be stored for 36 months at 36 °F to 46 °F (2 °C to 8 °C) from the date of manufacture; Within its shelf life, PANZYGA may be stored at room temperature (≤77 °F or 25 °C) for up to 12 months panzyga. After storage at room temperature (≤77 °F), product must be used or discarded; Do not freeze.. PANZYGA® (Immune Globulin Intravenous (Human)-ifas, 10% solution .. Support Frequently asked questions Pfizer PANZYGA Co-Pay Program for patients Ig Companion patient mobile app Frequently asked questions Request a representative Frequently asked questions about PANZYGA What are the most common adverse reactions (ARs) seen with PANZYGA?. CIDP in adults 1 The most common ARs reported in greater than 5% of subjects during a clinical trial were headache (15%; 21 .. Panzyga 100 mg/ml solution for infusion - Summary of Product .. Panzyga does not contain sucrose, maltose or glucose. Aseptic meningitis syndrome (AMS) Aseptic meningitis syndrome has been reported to occur in association with IVIg treatment. The syndrome usually begins within several hours to 2 days following IVIg treatment. Cerebrospinal fluid studies are frequently positive with pleocytosis up to several .. Pfizer recieves FDA approval for Panzyga to treat CIDP. This is the third indication for Panzyga, which was approved by the FDA in 2018 for the treatment of primary immunodeficiency in patients two years of age and older and chronic immune thrombocytopenia in adults. Our continued investment in supporting this important therapy reflects our ongoing dedication to advancing science across a spectrum .. Final results from the ProCID study of efficacy and safety of 3 .. The study enrolled 142 patients from 25 sites in 9 countries panzyga. The results of the study confirmed the efficacy of Panzyga® in adults with CIDP at the standard dose of 1.0 g/kg every 3 weeks. Almost 80% (55/69) of patients responded to treatment with a decrease of at least 1 point in the adjusted inflammatory neuropathy cause and treatment .. Immune globulin intravenous, human - ifas (Panzyga panzyga. - NC Medicaid panzyga. Effective with date of service Oct. 16, 2018, the NC Medicaid and Health Choice programs cover immune globulin intravenous, human - ifas (Panzyga®) for use in the Physician Administered Drug Program when billed with HCPCS code J1599 - Injection, Immune Globulin, Intravenous, Non-lyophilized (e.g. liquid), Not Otherwise Specified, 500 mg.. PANZYGA® (Immune Globulin Intravenous (Human)-ifas, 10% solution .. PANZYGA should be started at 1 mg/kg/min and continued at the infusion rates stated here, if tolerated 1; The example shown above illustrates a ramp-up schedule for an 80-kg patient using rates of 1, 2, 4, 8, and 12 mg/kg/min; For patients at risk of renal dysfunction or thromboembolic events, administer PANZYGA at the minimum infusion rate . panzyga
matibabu ya bawasiri
. PANZYGA® (Immune Globulin Intravenous (Human)-ifas, 10% solution .. INDICATIONS AND USAGE PANZYGA (Immune Globulin Intravenous [Human] - ifas) is indicated for the treatment of primary humoral immunodeficiency (PI) in patients 2 years of age and older; this includes, but is not limited to, congenital agammaglobulinemia, common variable immunodeficiency, X-linked agammaglobulinemia, Wiskott-Aldrich syndrome, and severe combined immunodeficiencies; chronic .. PANZYGA® (Immune Globulin Intravenous (Human)-ifas, 10% solution .. PANZYGA is a liquid medicine for infusion that contains immunoglobulin G (IgG), which are proteins that help fight infection. It is made from human plasma that is donated by healthy people and contains antibodies. For patients with PI, PANZYGA helps replace the missing antibodies in the body.. Randomized trial of three IVIg doses for treating chronic inflammatory .. IVIg (Panzyga ®, Octapharma) is a glycine-stabilized 10% liquid human IVIg 15, 16 with proven efficacy and safety as replacement therapy in primary immunodeficiency 17 and for immunomodulation in patients with immune thrombocytopenic purpura. 18 This IVIg product is licensed for different indications (including CIDP) in the USA, 19 Canada 20 .. Article - Billing and Coding: Immune Globulin (A57778) panzyga. Explanation of Revision: Based on a review, this billing and coding article was revised to add the new FDA approved drug Panzyga ® (immune globulin intravenous, human - IFAS) (HCPCS codes C9399/J1599) to the "CPT/HCPCS Codes/Group 1 Paragraph:/Group 1Codes:" and "ICD-10 Codes that Support Medical Necessity/Group 1 Paragraph:" sections.. PDF Drug Therapy Guidelines Immune Globulins Intravenous: Asceniv, BIVIGam .. o J1599 - Panzyga (500mg per 1 billable unit) o J1558 - Xembify (100 mg per 1 billable unit) o J3590 - Unclassified biologics (Cutaquig) VIII. Summary of Policy Changes . Drug Therapy Guideline Immune Globulins Last Review Date: 8/2022 Page 6 of 9 • 6/1/11:. Efficacy and safety of a new intravenous immunoglobulin (Panzyga. IVIG 10% (Panzyga ®; Octapharma AG, Wien, Österreich) is a novel high‐purity, glycine‐stabilised, liquid ready‐to‐use human immunoglobulin 10% (100 mg mL −1) IVIG product. It is manufactured using various precipitation and chromatography techniques for the harvesting and purification of immunoglobulin G. panzyga. PANZYGA Dosage & Rx Info | Uses, Side Effects - MPR. Store Panzyga for 36 months at +2°C to +8°C (36°F to 46°F) from the date of manufacture. Within its shelf-life, the product may be stored at ≤ +25°C (77°F) for up to 12 months. panzyga. PDF PANZYGA— Strength to move forward - gbs-cidp.org. PANZYGA is administered at home, in a hospital, or at an infusion center by a healthcare professional. They will start your infusion at a slow rate and increase the rate if you tolerate it well. The healthcare professional will be looking for signs of infusion reactions and may slow or stop your PANZYGA treatment if you. PANZYGA® (Immune Globulin Intravenous (Human)-ifas, 10% solution .. About Biochemical composition and manufacturing Storage and handling Vial sizes and NDC numbers Biochemical composition and manufacturing PANZYGA is a solvent/detergent-treated, sterile preparation of highly purified immunoglobulin G (IgG) 1 PANZYGA is a solution for infusion to be administered intravenously; PANZYGA is derived from large pools of human plasma. Panzyga (Immune Globulin Intravenous, human - RxList. What Is Panzyga? Panzyga (immune globulin intravenous- human solution) is an immune globulin intravenous (human) - is a 10% liquid preparation indicated for the treatment of primary humoral immunodeficiency (PI) in patients 2 years of age and older, chronic immune thrombocytopenia (ITP) in adults, and chronic inflammatory demyelinating polyneuropathy (CIDP) in adults. panzyga. panzyga® - Octapharma AG. What can we help you find? About us panzyga. About us . Who we are. Efficacy and Safety of Human Intravenous Immunoglobulin 10% (Panzyga .. Indeed, panzyga® could be safely administered at infusion speeds that were equal or considerably higher than used in most clinical trials treating antibody-deficient patients with IVIG, exceeding the maximum approved rates for most IVIG preparations [11, 17, 18]. Most patients (>90%) in the extension study tolerated the highest infusion speed .. PDF With the Pfizer PANZYGA Co-Pay Program - gbs-cidp.org. • PANZYGA is made from human blood and therefore may have a risk of transmitting infectious agents, including viruses and, theoretically, the variant Creutzfeldt-Jakob disease (CJD) and CJD agent. The production and manufacturing process reduces this risk, but the risk cannot be eliminated $0 2. panzyga. PANZYGA (immune globulin intravenous- human solution - DailyMed panzyga. PANZYGA contains mainly immunoglobulin G (IgG) with a broad spectrum of antibodies against various infectious agents reflecting the IgG activity found in the donor population. PANZYGA which is prepared from pooled material from not less than 1000 donors, has an IgG subclass distribution similar to that of native human plasma. .. RETIRED - Panzyga® (Immunoglobulin Intravenous (Human), 10% . - Noridian. Panzyga® (Immunoglobulin Intravenous (Human), 10%) is a sterile liquid preparation of highly purified immunoglobulin G (IgG) derived from large pools of human plasma. The FDA approved Panzyga® on August 2, 2018. Panzyga® is covered for claims with dates of service on or after August 2, 2018 when the criteria below are met. .. Pfizer Introduces Warranty Program for Rare Disease Drug Panzyga. Panzyga, which won FDA approval in 2021, is an intravenous immunoglobulin (IVIg). The Pfizer Pledge Warranty Program is available to adult, cash-paying or commercially insured patients panzyga. Patients are not eligible for the program if their Panzyga was covered, in whole or in part, by Medicare, Medicaid, TRICARE, Veterans Affairs healthcare, a .. Efficacy and Safety of Human Intravenous Immunoglobulin 10% (Panzyga . panzyga. Purpose: To assess the efficacy and safety of panzyga® (intravenous immunoglobulin 10%) in preventing serious bacterial infections (SBIs) in patients with primary immunodeficiency diseases (PIDs), a prospective, open-label, multicenter, phase 3 study and an open-label extension study were undertaken. Methods: Initially, the study drug (infusion rate ≤0.08 mL/kg/min) was administered at .. Immune Globulin-Ifas (Intravenous Route) - Mayo Clinic. Panzyga; Descriptions. Immune globulin-ifas injection contains antibodies that make your immune system stronger. It is used for patients who have primary humoral immunodeficiency (PI), including congenital agammaglobulinemia, common variable immunodeficiency, X-linked agammaglobulinemia, Wiskott-Aldrich syndrome, and other severe combined .. LCD - Intravenous Immune Globulin (L33610) - Centers for Medicare .. The statutory coverage criteria for intravenous immune globulin (IVIG) addressed in this policy are specified in the related Policy Article
harga tayar kereta terkini
. For the items addressed in this LCD, the "reasonable and necessary" criteria, based on Social Security Act § 1862 (a) (1) (A) provisions, are defined by the following coverage indications, limitations . panzyga. Randomized trial of three IVIg doses for treating chronic . - PubMed. The Progress in Chronic Inflammatory Demyelinating polyneuropathy (ProCID) study was a prospective, double-blind, randomized, parallel-group, multicentre, phase III study investigating the efficacy and safety of 10% liquid intravenous immunoglobulin (Panzyga®) in patients with active chronic inflammatory demyelinating polyneuropathy. panzyga. PDF Immune Globulin Reference Chart - FFF Enterprises. 6 months without return to refrigeration panzyga. After storage at 25°C (77°F), discard if not used. Do not freeze. Do not shake solution. 2°C to 8°C (36°F to 46°F) for up to 36 months, or room. Article - Billing and Coding: Immune Globulin (A56786) panzyga. Inj, panzyga, 500 mg Group 2 (8 Codes) Group 2 Paragraph. Subcutaneous formulations of immune globulin. When billing HCPCS code J1561 or J1569, append the JB modifier for the subcutaneous formulation. Group 2 Codes. Code Description; J1551 Inj cutaquig 100 mg .. Immunoglobulin replacement therapy | Immune Deficiency Foundation. Immunoglobulin (Ig) replacement therapy is the standard treatment for individuals with PI who lack antibodies, also known as immunoglobulins, or whose antibodies do not function properly. These types of PI, such as common variable immunodeficiency (CVID) and X-linked agammaglobulinemia (XLA), are known as antibody deficiencies. panzyga. Pfizer Reports Strong First-quarter 2021 Results. Panzyga (immune globulin intravenous [human] ifas 10% liquid preparation)-- In February 2021, Pfizer announced that the FDA approved the supplemental Biologics License Application (sBLA) for .
sakonin soyayya
. PDF HPHC Chemotherapy HCPCS J-Codes List Prior Authorization Required. PANZYGA 10% (5 G/50 ML) VIAL J9229 Inotuzumab ozogam inj, 0.1 mg Inotuzumab Ozogamicin BESPONSA 0.9 MG VIAL A9699 Iodine i-131 iobenguane tx,1 millicurie Iobenguane Iodine 131 AZEDRA THERAPEUTIC VIAL J9228 Ipilimumab inj, 1 MG Ipilimumab YERVOY 50 MG/10 ML VIAL J9206 Irinotecan inj, 20 MG Irinotecan Hcl IRINOTECAN HCL 500 MG/25 ML VL J9205 panzyga. Panzyga Alternatives Compared - Drugs.com panzyga
турецкий сериал кровавые цветы
. Panzyga has an average rating of 8.0 out of 10 from a total of 1 ratings on Drugs.com. 100% of reviewers reported a positive effect, while 0% reported a negative effect. Promacta has an average rating of 7.1 out of 10 from a total of 17 ratings on Drugs.com. 64% of reviewers reported a positive effect, while 21% reported a negative effect panzyga. . panzyga. Efficacy and safety of a new intravenous immunoglobulin (Panzyga - PubMed panzyga. Objectives: To assess the efficacy and safety of intravenous immunoglobulin (IVIG) 10% (Panzyga ®), a novel human normal IVIG 10%, in patients with chronic immune thrombocytopenia (ITP) panzyga. Background: First-line treatment options in ITP include IVIGs. Methods: In this prospective, open-label, non-controlled, multicentre, phase III study, patients received a daily dose of IVIG 10% (1 g kg-1 body .. PDF What Panzyga is and what it is used for - medicines. Panzyga may impair the effect of live attenuated virus vaccines such as • measles • rubella • mumps • varicella panzyga. After administration of this product, an interval of 3 months should elapse before vaccination with live . attenuated virus vaccines. In the case of measles, this impairment may persist for up to 1 year.. Panzyga Prices, Coupons, Copay & Patient Assistance - Drugs.com panzyga. Panzyga Prices, Coupons and Patient Assistance Programs

sa vjec eshte xhemi shehu
. Vacancies. Human Normal Immunoglobulin.. Panzyga (immune globulin intravenous, human - ifas) | CenterWatch. Panzyga is an immune globulin intravenous (human) - ifas 10% liquid preparation. Panzyga supplies a broad spectrum of opsonic and neutralizing IgG antibodies against bacteria or their toxins. The mechanism of action in PI has not been fully elucidated. Treatment of ITP and CIDP in Adults : The mechanism of action of immunoglobulins in adults .. PANZYGA® (Immune Globulin Intravenous (Human)-ifas, 10% solution) | Ig .
. PP-PAN-USA-0594. INDICATIONS AND USAGE PANZYGA (Immune Globulin Intravenous [Human] - ifas) is indicated for the treatment of primary humoral immunodeficiency (PI) in patients 2 years of age and older; this includes, but is not limited to, congenital agammaglobulinemia, common variable immunodeficiency, X-linked agammaglobulinemia, Wiskott ..